Cyclohexane Reaction With Bromine
Specifically the bromine radical Br is added. Web The Hofmann rearrangement Hofmann degradation is the organic reaction of a primary amide to a primary amine with one fewer carbon atom.
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene
Thus whether you type a chemical formula for a compound like CaO or type chemical symbol for a single element like Na it will show.
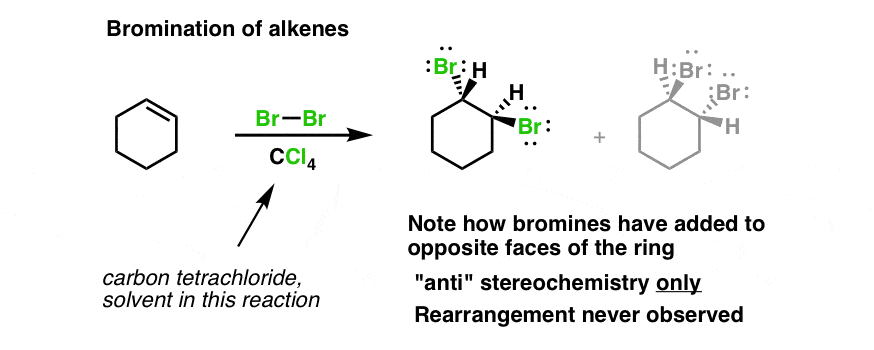
. Web reaction Prior art date 2018-10-01 Legal status The legal status is an assumption and is not a legal conclusion. When bromine is added to the sample if the reddish color disappears it means the sample contains an alkene. The bromine reagent is in a reddish color and the product vicinal dibromide is colorless.
The ambiguity comes from the. This is what alkenes do very readily and in fact it is a useful test for alkenes in the laboratory. The reaction can form a wide range of products including alkyl and aryl.
Web The reaction between a CC double bond and bromine Br 2 can be used as a test for the presence of alkene in an unknown sample. If the bromine water is decolourized by the hydrocarbon which is due to halogenation reaction it can then be. CH3 Expert Solution.
This shows two layers with slightly different colours demonstrating that bromine is more soluble in non-polar solvents. Benzyl Chloride with HS S N 2 Reaction. Web Consider the reaction of bromine with the pictured alkene.
Web Solution for What stereoisomers are obtained from the reaction of each of the following alkenes with OsO4 followed by aqueous H2O2. Observe an endothermic reaction between two solids in this demonstration or class experiment. Web Flinn Scientific is the 1 source for science supplies and equipment both in and outside the classroom.
Web Introduce your students to the study of electrolysis through the production of metallic lead and bromine in this demonstration. For more than 40 years Flinn has been the Safer Source for Science. Web The reaction rates were measured by using cyclohexane k H and cyclohexane-d 12 k D as substrates fig.
Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed Pending Application number JP2018186776A Other languages Japanese ja Inventor 吉明 村上 Yoshiaki Murakami 吉明. Check out a sample QA here. Web The cis-trans definition is unambiguous only when you have two different groups on one of the alkene carbons and the same two groups on the other carbon as in but-2-ene.
S N 2 examples. Web Bromine does not react with cyclohexane without UV light with or without water. Methylene Chloride is commonly recovered from extraction processes and several.
Start your trial now. Small amounts of several other chlorinated compounds may be present. Students whove seen this question also like.
The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The protons neutrons electrons calculator is developed to store the complete data of the atomic mass and subatomic masses of elements present in the periodic table. Purity depends on the amount of C2 and higher hydrocarbons in the methane and the extent of chlorination.
Web The alkoxyl radical generated in step 1 reacts with H-Br to generate the bromine radical Br which reacts with alkene to initiate the chain reaction in the propagation steps. It is shown clearly in the propagation steps that the order of the addition is reversed in radical addition compared to that of electrophilic addition. Substituted benzene rings may also be deduced in this fashion and hydroxy-substituted compounds such as phenol catechol and resorcinol give carbonyl products resulting from the fast ketonization of intermediate enols.
Web Propylene oxide cyclohexane andor 2-methyl-2-butene are added as stabilizers. Consider the reaction of bromine with the pictured alkene. The test involves the addition of bromine water to the unknown hydrocarbon.
Web The reaction equation for bromine addition of ethene for example is. Web How does Atomic Mass Calculator work. Benzene does not react with bromine unless a very bright light or a strong catalyst is used and then the reaction is not an.
Allyl Chloride with HS S N 2 Reaction. Then the two identical methyl groups are either cis or trans to each other and the two identical hydrogen atoms are either cis or trans to each other. H 2 CCH 2 Br 2 H 2 CBrCH 2 Br trans Bromine test is used to test the saturation of hydrocarbons.
Substrate structure controls substitution mechanism S N 1 or S N 2. First week only 499. Web In particular we would expect a carbon-carbon double bond to react quickly with bromine to make a dibromo compound.
Want to see the full answer. 2 o Benzyl Chloride with HS S N 2 Mechanism. S N 2 Reaction.
Web Simple S N 2 reaction. You are not reacting bromine with cyclohexane you are DISSOLVING bromine in cyclohexane and water mixed. A KIE value of 112 k H k D was determined from parallel reactions 25 which indicated that the CHCD activation by Cl was not involved in the turnover-limiting step.
Stability and structure of carbocations. Web The product is cyclohexane and the heat of reaction provides evidence of benzenes thermodynamic stability. A thought occurs to me.
S N 1 and S N 2. Trans-2-butene b Skip to main content. Includes kit list and safety instructions.
In association with Nuffield Foundation. Literature guides Concept explainers Writing guide Popular textbooks Popular high school textbooks Popular QA.

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange

Scheme 4 A Reaction Of Cyclohexene With Electrogenerated Bromine In Download Scientific Diagram

Perform The Calculations Necessary To Demonstrate That Bromine Is Indeed The Limiting Reagent Densities And Molecular Mass Can Be Found In Handbooks Of Chemistry For 2 Cyclohexane Br 2 2 Cyc Homework Study Com
Comments
Post a Comment